Tivozanib
FOTIVDA® (tivozanib) is an oral, once-daily, next-generation vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor (TKI). It is a potent, selective inhibitor of VEGFRs 1, 2, and 3 with a long half-life designed to improve efficacy and tolerability.1-3
Tivozanib in RCC
AVEO received U.S. Food and Drug Administration approval for FOTIVDA in March 2021 for the treatment of adult patients with relapsed or refractory advanced renal cell carcinoma (RCC) following two or more prior systemic therapies.1 FOTIVDA was approved in August 2017 in the European Union and other countries in the territory of its partner EUSA Pharma for the treatment of adult patients with advanced RCC.2 Tivozanib has been shown to significantly reduce regulatory T-cell production in preclinical models.4 Tivozanib was discovered by Kyowa Kirin.
AVEO and Bristol-Myers Squibb Company (“BMS”) are collaborating on a Phase 3 clinical trial designed to evaluate the safety and efficacy of tivozanib in combination with OPDIVO® (nivolumab) as compared to tivozanib as a monotherapy, in RCC patients who have progressed following prior immune checkpoint inhibitor therapy, known as the TiNivo-2 trial. For more information, please visit www.TiNivo2.com.
Tivozanib in Other Indications
AVEO has presented positive data from its Phase 1b/2 Study of tivozanib in combination with IMFINZI (durvalumab), AstraZeneca’s human monoclonal antibody directed against programmed death-ligand 1 (PD-L1), in patients with unresectable locally advanced or metastatic, previously untreated hepatocellular carcinoma (HCC). The rationale for a combination therapy of tivozanib plus durvalumab to treat HCC draws on the potential synergistic mechanisms of tivozanib and durvalumab to remove inhibition of the immune response that mediates anti-tumor activity.
AVEO is supporting an Investigator Sponsored Trial with the University of Florida to conduct a Phase 1b/2clinical trial to evaluate the safety and efficacy of tivozanib in combination with atezolizumab in multiple immunologically cold tumors, including pancreatic, gallbladder and biliary cancers. Immunologically cold tumors are solid tumors that lack or have few tumor-infiltrating lymphocytes and remain a clinical challenge.
AVEO has a Cooperative Research And Development Agreement with the National Institutes of Health to conduct a Phase 1/2 clinical trial designed to evaluate the safety and efficacy of tivozanib in cholangiocarcinoma (CCA). The trial is an open-label, single-center, non-randomized study which enrolled its first patient in March 2022. CCA is an aggressive biliary tract malignancy that remains a clinical challenge with limited treatment options and poor survival rates.
Data from an investigator-sponsored Phase 2 clinical trial of tivozanib in patients with recurrent, platinum-resistant ovarian cancer, including fallopian tube or primary peritoneal cancer, was presented at the 2019 ASCO Annual meeting. The trial was one of several studies funded by a grant AVEO provided to the National Comprehensive Cancer Network and was designed to measure the safety and activity of tivozanib in ovarian cancer. Out of the 31 patients enrolled in the study, 30 were treated with tivozanib, four patients showed a partial response and twelve patients had stable disease. The clinical benefit rate (partial response plus stable disease) was reported to be 53.3%, suggesting that tivozanib is active in patients with recurrent ovarian cancer, without substantial toxicity.
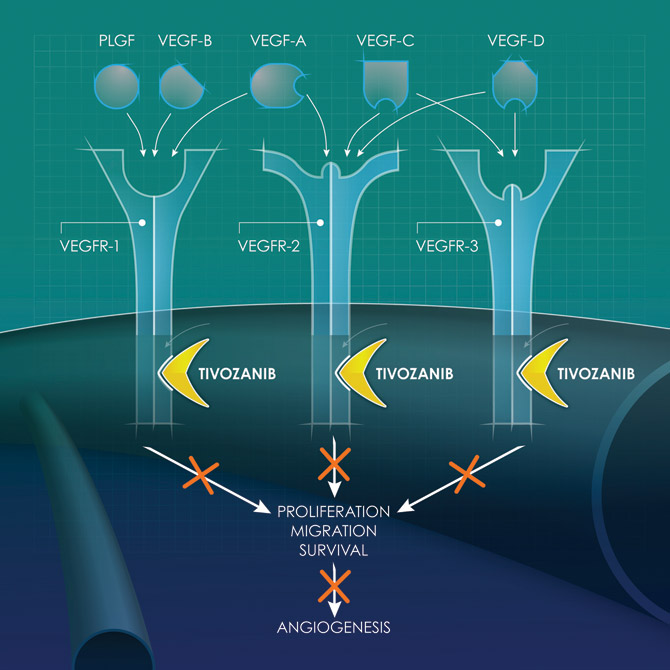
VEGF As a Therapeutic Target
The vascular endothelial growth factor (VEGF) pathway plays a significant role in angiogenesis, which is critical in cancer. Angiogenesis, the formation of new blood vessels, is essential for endothelial cell proliferation, migration and survival. There are five known VEGF ligands (A, B, C, D, PLGF) and three VEGF receptors (1, 2 and 3). Each ligand exhibits distinct but overlapping binding profiles for the three VEGF receptors.
To optimally inhibit the VEGF pathway it may be critical to effectively block all three VEGF receptors as each plays an important role in cancer angiogenesis:
- VEGF receptor 1 is critical for the modulation of endothelial cell survival and vessel morphogenesis;
- VEGF receptor 2 is thought to be the predominant receptor for endothelial cell proliferation and migration; and,
- VEGF receptor 3 promotes endothelial sprouting and vascular network formation.
Preclinical studies have demonstrated tivozanib is a potent, selective, long half-life inhibitor of all three VEGF receptors that is designed to optimize VEGF blockade while minimizing off-target toxicities.
For more information, please visit FOTIVDA
1FOTIVDA (Tivozanib) USPI March 2021.
2FOTIVDA (Tivozanib) SmPC August 2017.
3Motzer RJ, Nosov D, Eisen T, et al. J Clin Oncol 2013; 31(30): 3791-9.
4Pawlowski N et al. AACR 2013. Poster 3971